On October 9th, the Royal Swedish Academy of Sciences announced today that it will award the 2019 Nobel Prize in Chemistry to John B, a professor at the University of Texas at Austin. Goodenough, Professor of the State University of New York at Binghamton. Stanley Whittingham, and Professor Yoshino Akira of the University of Japan, in recognition of their "contributions in the process of inventing lithium batteries."
They created a rechargeable world
The 2019 Nobel Prize in Chemistry rewards the invention of lithium batteries. This lightweight, rechargeable and powerful battery has already entered the homes of ordinary people today, and is used by every mobile phone, notebook and other electronic devices. It can also be used to store solar and wind energy, making it possible to build a society where zero fossil fuels are used.
Lithium batteries are widely used worldwide to power portable electronic devices, and we communicate, work, research, listen to music, or retrieve knowledge. The invention of lithium batteries also makes it possible to develop electric vehicles that can travel over long distances, and it is also widely used in renewable energy sources such as solar and wind energy storage.
Lithium-ion batteries have revolutionized our lives and are used in everything from cell phones to laptops and electric cars. Through their work, this year's chemistry winners laid the foundation for a wireless, fossil fuel-free society.
The basis for the development of lithium batteries was built during the oil crisis of the 1970s. At the time, Stanley Whittingham was working on an energy technology that could get rid of petroleum fuels. He began researching superconductor materials and quickly discovered an extremely versatile material that he used to creatively make cathodes for lithium batteries. This is made using titanium disulfide, and at the molecular level, the internal voids can accommodate lithium ions.
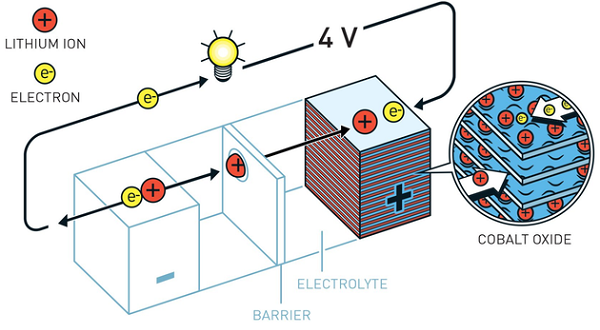
The positive portion of the battery is made of metallic lithium. Lithium has a strong driving force for releasing electrons. This formed a battery with a huge potential, just over 2 volts. However, metallic lithium is active and the risk of battery explosion is too high to be commercially viable.
John Goodenough predicts that if a metal oxide is used instead of a metal sulfide to make the cathode, the battery will have a higher potential. After systematic research, in 1980, he demonstrated that cobalt oxide embedded in lithium ions can generate voltages of up to 4 volts. This is an important breakthrough that will bring a more powerful battery.
In the early 1970s, when Stanley Whittingham (a winner of this year's Chemistry Prize) developed the first working lithium battery, he used the enormous power of lithium to release its external electronics.
Based on Goodenough's cathode, Yoshino Akatsu invented the first commercially viable lithium-ion battery in 1985. Instead of using active lithium at the anode, he uses petroleum coke, a carbon material that, like the cobalt oxide of the cathode, can be inserted into lithium ions.
As a result, the researchers obtained a lightweight and durable battery that can be charged hundreds of times before performance is exhausted. Lithium-ion batteries have the advantage that they are not based on chemical reactions of the decomposition electrodes, but rather flow back and forth between the positive and negative electrodes based on lithium ions.
Since it was first introduced to the market in 1991, lithium-ion batteries have revolutionized our lives. They have laid the foundation for wireless communications and the establishment of a society without fossil fuels, and have brought enormous benefits to mankind.