Against the background of energy crisis and environmental pollution, lithium-ion batteries, as an ideal energy source for development in the 21st century, have received more and more attention. However, some failure phenomena may occur in the production, transportation, and use of lithium-ion batteries. Moreover, the failure of a single battery will affect the performance and reliability of the entire battery pack, and even cause the battery pack to stop working or other safety issues.
Lithium-ion batteries often have certain failure phenomena during use or storage, including capacity decay, internal resistance increase, rate performance reduction, gas production, liquid leakage, short circuit, deformation, thermal runaway, lithium evolution, etc., which seriously reduce lithium ion The performance, reliability and safety of the battery. These failure phenomena are caused by the interaction of a series of complex chemical and physical mechanisms inside the battery.
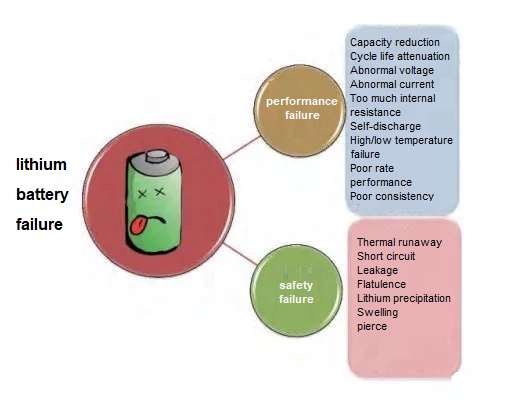
Classification of lithium battery failure
In order to avoid performance degradation and battery safety issues, it is imperative to carry out lithium battery failure analysis. Lithium battery failure refers to the degradation of battery performance or abnormal use performance caused by some specific essential reasons, and it is divided into performance failure and safety failure.
Causes of lithium battery failure
The reasons for the failure of lithium batteries can be divided into internal and external causes.
The internal cause mainly refers to the nature of the physical and chemical changes of the failure. The research scale can be traced back to the atomic and molecular scales to study the thermodynamic and dynamic changes of the failure process.
External factors include impacts, needle sticks, corrosion, high-temperature combustion, man-made damage and other external factors.
Common failure performance and failure mechanism analysis of lithium batteries
1. Capacity attenuation
It is mainly divided into two types: reversible capacity attenuation and irreversible capacity attenuation. Reversible capacity attenuation can restore the lost capacity by adjusting the battery charging and discharging system and improving the battery usage environment; irreversible capacity attenuation is an irreversible change in the battery's internal capacity that produces irreversible capacity loss.
The root cause of battery capacity decay failure is the failure of materials, and it is closely related to objective factors such as battery manufacturing process and battery use environment. From a material point of view, the main reasons for the failure are the structural failure of the positive electrode material, the excessive growth of SEI on the negative electrode surface, the decomposition and deterioration of the electrolyte, the corrosion of the current collector, and the trace impurities of the system.
2. Increased internal resistance
The internal resistance of lithium-ion batteries is related to the process of electron transmission and ion transmission within the battery system, and is mainly divided into ohmic resistance and polarization internal resistance. The internal resistance of polarization is mainly caused by electrochemical polarization, and there are two kinds of electrochemical polarization and concentration polarization. The main factors leading to the increase of the internal resistance of lithium-ion batteries are divided into key battery materials and battery use environment.
3. Internal short circuit
The performance of short circuit can be divided into the following four types:
a). Short circuit between copper/aluminum current collectors;
b). The diaphragm fails to lose electrical insulation or the gap becomes micro-contact between the positive and negative electrodes, and local heating occurs severely. During further charging and discharging, it may spread to the surroundings and cause thermal runaway;
c). Transition metal impurities in the positive electrode slurry are not cleanly removed, piercing the diaphragm, or promoting the generation of negative lithium dendrites, causing internal short circuits;
d). Lithium dendrites cause internal short circuits.
In addition, in the process of battery design and manufacturing or battery pack assembly, unreasonable design and excessive local pressure can also cause internal short circuit; induced by battery overcharge and overdischarge, internal short circuit will also occur, mainly due to the current collector Corrosion, deposition on the electrode surface, in severe cases, the positive and negative electrodes will be connected through the diaphragm.
4. Gas production
Li-ion battery gas production is mainly divided into normal gas production and abnormal gas production. During the battery formation process, the gas production phenomenon that occurs when the electrolyte is consumed to form a stable SEI film is normal gas production. The gas produced in the formation stage is mainly H2, CO2, C2H2, etc. produced by ester single/double electron reaction. Abnormal gas production is mainly caused by excessive consumption of electrolyte and oxygen release from positive electrode materials during the battery cycle. It often occurs in soft-pack batteries, causing excessive internal pressure and deformation of the battery, breaking the encapsulated aluminum film and internal Battery contact problems, etc.
5. Thermal runaway
Thermal runaway refers to the rapid increase in temperature inside the lithium-ion battery. The heat cannot be dissipated in time, and a large amount of heat accumulates inside and induces further side reactions.
In order to prevent lithium-ion batteries from causing serious safety problems in thermal runaway, measures such as PTC, safety valves, and thermally conductive films are often used. At the same time, systematic aspects such as battery design, battery manufacturing process, battery management system, and battery use environment are required considerations.
6. Lithium precipitation
Lithium precipitation is a relatively common phenomenon of aging and failure of lithium-ion batteries. The main manifestation is that a layer of gray, off-white or gray-blue material appears on the surface of the negative pole piece. These substances are metallic lithium precipitated on the surface of the negative electrode.


