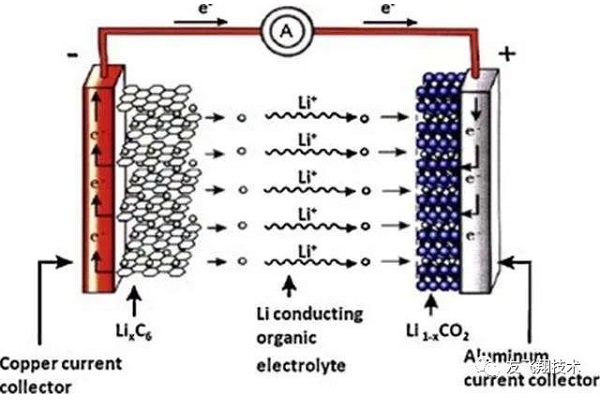
Lithium-ion batteries have been commercialized since the 1990s. Nowadays, they have entered the daily life of ordinary people. We use mobile phones, tablets, smart watches, VR glasses, digital cameras and other portable electronic products, almost all use lithium-ion batteries.
The lithium-ion battery itself also developed from the early aluminum and steel cases to flexible packaging. Due to the flexible packaging of lithium-ion batteries, the shape and size of the lithium-ion batteries are more flexible, and the safety performance is superior. Become as the favorite application battery.
However, it is an indisputable fact that lithium-ion batteries in flexible packaging are more likely to swell than aluminum or steel batteries.

The main reasons for the inflation of soft-pack lithium-ion batteries are:
1. Over-charge. In the over-charged state of lithium-ion batteries, the positive electrode (anode) material will release too many lithium ions, which causes its normal crystal structure to collapse, the electrochemical performance is unstable, and it is easy to generate oxygen at the positive electrode, which in turn will interact with the electrolyte. The solvent reacts to generate carbon dioxide and water, and the water will further react with the electrolyte solvent and lithium salt to generate more carbon dioxide and hydrofluoric acid.

2. Over-discharge. In the over-discharged state of lithium-ion batteries, SEI (Solid-Liquid Phase Membrane) will decompose with the electrolyte solvent to generate alkane gas, which will cause the battery to swell, especially after the battery continues to charge after over-discharge, the negative electrode and electrolyte will regenerate new SEI membranes will also produce alkanes.
3. High temperature. The upper limit temperature for lithium ion batteries is usually 55 ~ 60 ° C, and the upper limit temperature for some special high temperature batteries reaches a high temperature of 80 ~ 85 ° C. If the lithium ion battery exceeds the upper limit temperature, the internal electrolyte will decompose and produce gas, SEI (Solid-Liquid Phase Membrane) will decompose and produce gas, and SEI (Solid-Liquid Phase Membrane) regeneration process will also produce gas.
4. Water intrusion. The packaging film of the soft pack lithium-ion battery is an aluminum-plastic film, which is mainly composed of an inner layer of CPP, a middle layer of Al, and an outer layer of nylon. The thickness is about 0.088 to 0.152 mm. Its main function is to protect the lithium ion battery from the invasion and insulation of external moisture and oxygen. The electrode tabs of soft the lithium-ion battery is mainly composed of a metal strip and an tab gel. The electrode tabs gel and the CPP layer of aluminum plastic film are fused at high temperature to achieve the sealing effect. If the aluminum-plastic film is damaged, poorly packaged, electrochemically corroded, and the shorts of the tabs cause local high temperatures to cause the metal strip to separate from the tabs, external moisture will enter the battery at this time, causing the battery to swell.
Regarding electrochemical corrosion, the basic principle is that the negative electrode forms an electronic path with the aluminum-plastic film aluminum layer, and at the same time, the aluminum-plastic film aluminum layer and the electrolyte directly form an ion path. Together, the two make Cu // electrolyte // Al form a primary cell. The electrode potential of Al is lower than that of the negative electrode Cu. The Al layer as the anode of the primary cell loses electrons and becomes Al3 +, which is dissolved in the electrolyte. From the outside, there are a lot of black spots at the edge of the aluminum-plastic film, which is the phenomenon that the Al layer is corroded. The battery loses the barrier of oxygen and moisture to the aluminum-plastic film, and bloating occurs.
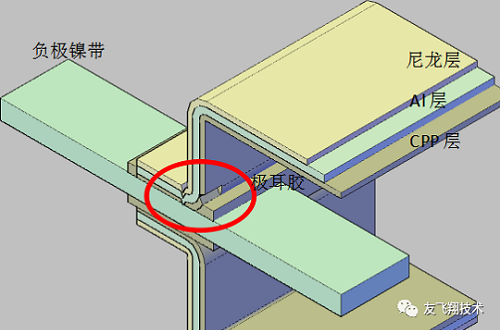
Generally, electronic conduction is easy to occur in the battery top-sealing process; ion conduction is easy to occur when the aluminum-plastic film is stamped and thermally damaged to CPP. The former can be screened by resistance test after encapsulation, while the latter has no suitable method for screening in the production process. Therefore, it is necessary to avoid contact between the negative electrode in the external circuit and the aluminum layer at the aluminum-plastic film fault during use to prevent electrochemical corrosion.
In summary, when using lithium-ion batteries, we need to use appropriate protection circuits to avoid overcharging, overdischarging, and short-circuiting of the battery, and try to avoid the surface of the battery contacting sharp objects to cause scratches on the aluminum-plastic film Injuries that can effectively prevent battery inflation.


